the tendency of an atom to attract electrons towards itself
The tendency of an atom to pull electrons towards itself is referred to as its electronegativity. Electronegativity is the property of an atom involved in a covalent bond to withdraw the shared pair of electrons towards itself.
 |
| Define The Term Electronegativity State Its Unit |
Is the strength of an atom to attract the electrons towards itself in a bond means if one of the atoms has more tendency to attract electrons it is said to be more.
. The chlorine atom in HCl develops a partial negative charge and hydrogen atoms develop a. The tendency of an atom to attract bonding electrons to itself from another atom is referred to as electronegativity. Electronegativity describes the tendency of an atom to attract the shared electrons in a bond towards itself. Electronegativity is defined as the ability of an atom to attract electrons toward itself in a chemical bond.
The tendency for an atom of an element to attract electrons to itself when it is chemically combined with another element. If atoms bonded together have the. An atoms electronegativity is affected by both its. Electronegativity is tendency of an atom to attract a shared pair of towards itself.
Large differences in electronegativity between atoms in a given molecule often cause the complete transfer of an. Electronegativity In this molecule what type of bond is found between the oxygen and hydrogens. Ii The process by which certain ores specially carbonates are converted. The electronegativity of an atom depends on the following.
The correct option is A electrons The tendency of an atom in a molecule to attract the shared pair of electrons towards itself is known as electronegativity. One Line Answer The tendency of an atom to attract electrons itself when combined in a covalent compound. It is the ability of the atom in covalent molecule to attract the. Electronegativity Definition Electronegativity is a chemical property that measures the tendency of an atom to attract electrons towards itself.
Electronegativity is the measure of the tendency of an atom to attract greater electronegativity electrons towards itself. It is the tendency of an atom to attract bonding pair of electrons towards itself. Electronegativity is the tendency of an atom to attract electrons in a molecule. The tendency of an atom to pull electrons towards itself is called electronegativity.
The tendency of an atom to attract electrons towards itself. The relative tendency of an atom in a molecule to attract the shared pair of electrons towards itself is called electronegativity. When it comes to chemical properties electronegativity is use to describe the. Higher electronegativity results in stronger attraction.
How big of an e hog an atom is. This concept is related to both Electron Affinity and Ionization Energy two of. The tendency of an atom to pull electrons toward itself is referred to as its _____. Of the two elements Pb and Sb has the core Pb core Sb nonbonding.
Atoms with higher electronegativity have a higher tendency to attract electrons towards itself. So in a bond the shared. Advertisement Remove all ads Solution Electronegativity Concept. What does the electronegativty of an atom indicate.
Electronegativity of an atom is its tendency to attract a pair of electrons in bond. I The tendency of an atom to attract electrons towards itself when combined in a covalent compound. For example in a chemical bond C-F Fluorine has a tendency. Electronegativity is affected by the.
Electronegativity is a property that describes the tendency of an atom to attract electrons or electron density toward itself.
 |
| 6 3 Covalent Bonding Chemistry Libretexts |
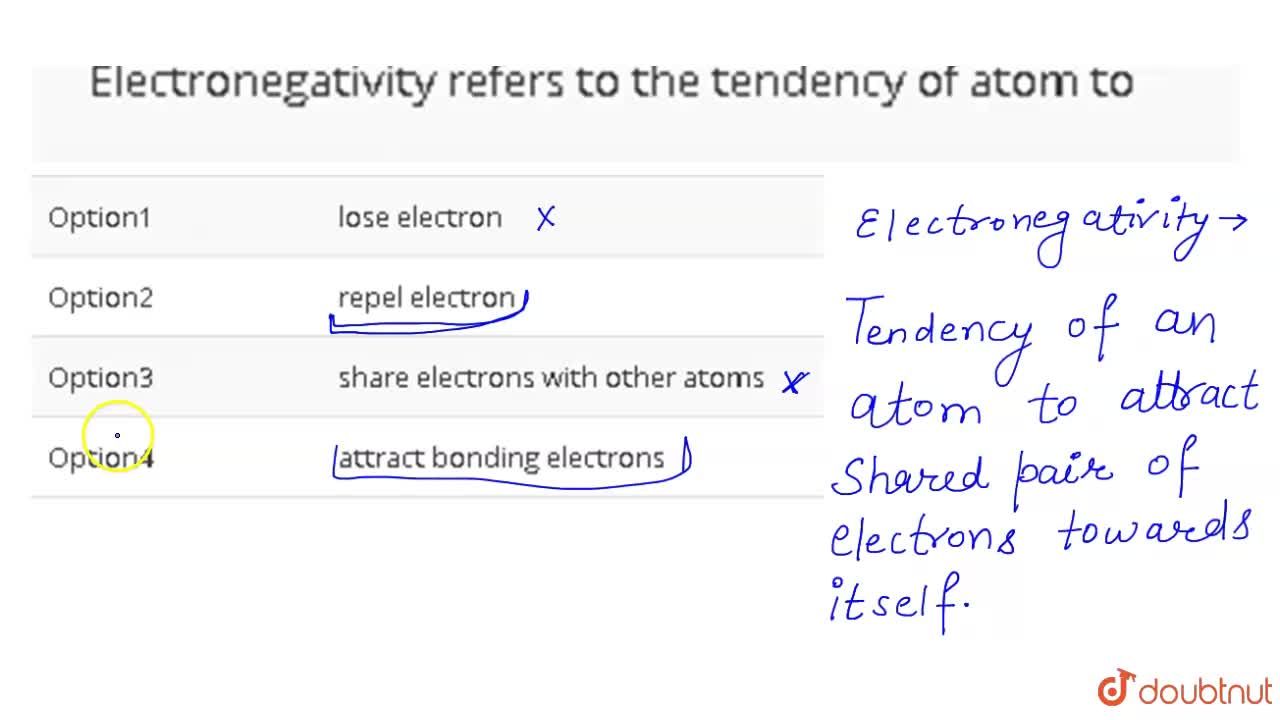 |
| Electronegativity Refers To The Tendency Of Atom To |
 |
| Is It A Tendency Of An Atom To Attract Electrons Quora |
 |
| Electronegativity Polarity Interaction And Resonance Concise Medical Knowledge |
 |
| Molecular Interactions Analytical Chemistry Lecture 2 Molecular Interactions Molecular Studocu |
Posting Komentar untuk "the tendency of an atom to attract electrons towards itself"